癌症中的RET融合检测
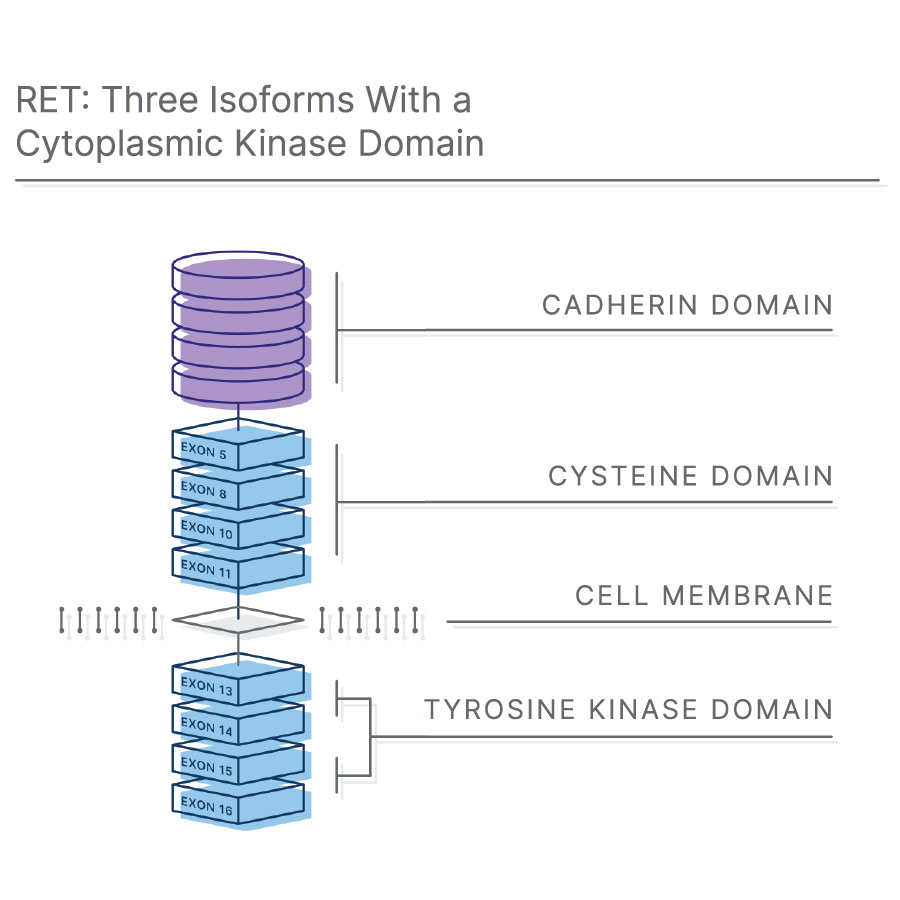
什么是RET融合?
RET融合会导致一种与多种癌症发病机制相关的癌基因被激活1-4。已知RET是至少12种基因的融合伴侣,其中KIF5B-RET是NSCLC中最常见的RET融合5,6。
RET(转化过程中发生重排)基因编码跨膜受体酪氨酸激酶7。RET是胶质细胞系源性神经营养因子家族配体(GFL)的受体,GFL是一组可溶性神经营养因子,在胚胎形成和人类发育过程中具有重要作用8,9。
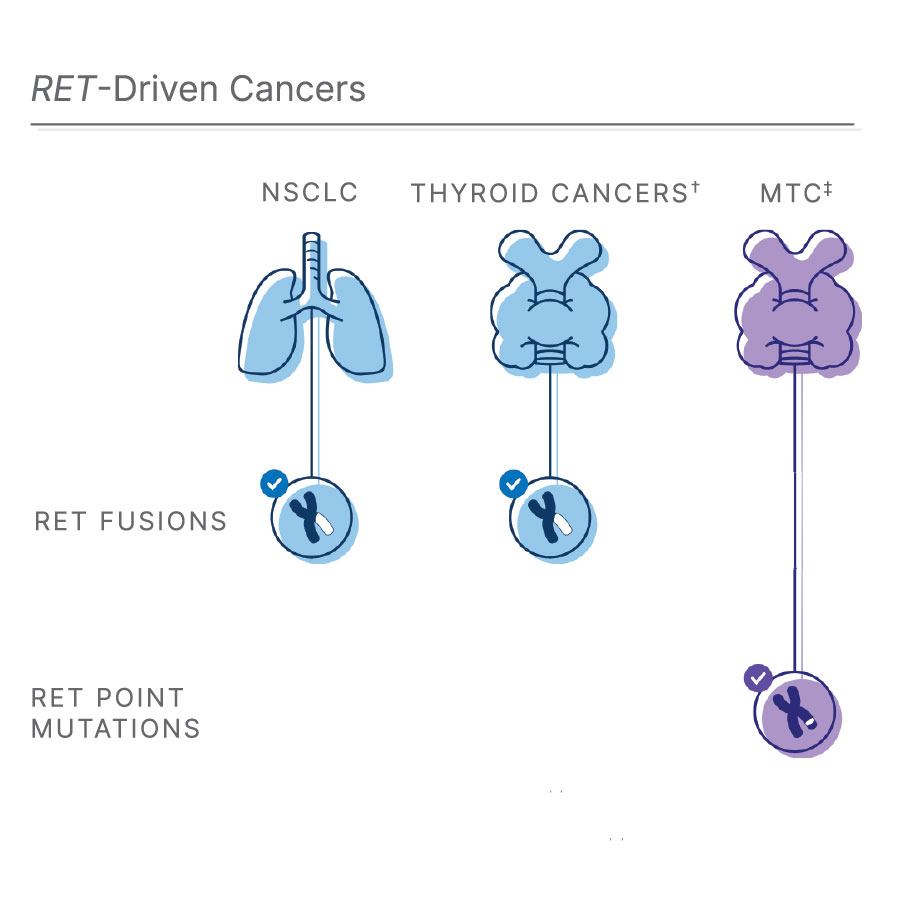
†RET融合也见于10%-20%的甲状腺乳头状癌(PTC)1, 11, 12
‡甲状腺髓样癌:RET点突变会影响大多数MTC1, 3, 12
RET融合是常见肺癌类型的生长驱动因子
每年,罹患肺癌的人数超过200万,其中85%的患者为非小细胞肺癌(NSCLC)10。在这些NSCLC患者中,约2%与RET融合有关1,8。此外,10%-20%的甲状腺乳头状癌(PTC)也存在RET融合1,11,12。
虽然RET突变十分罕见,但新疗法使其具有重要意义。FDA批准RET选择性治疗药物RETEVMO®(selpercatinib)和GAVRETO® (pralsetinib)后, RET成为转移性NSCLC和甲状腺癌患者需要检测的重要生物标志物。此外,随着RETEVMO® (selpercatinib)在泛肿瘤领域的获批,RET融合检测让晚期或转移性实体瘤患者有了更多治疗选择。因此,为了实现创新精准医疗,必须对所有有意义的生物标志物进行检测。
使用全景变异分析检测罕见生物标志物
在一项针对1万例患者开展的研究中,37%的患者通过CGP检测出了有意义的突变14。鉴于目前已有针对RET融合的靶向疗法,对NSCLC进行分子表征的需求进一步扩大。
通过DNA + RNA工作流程,CGP能够在单次检测中同时评估更多常见和罕见驱动突变。通过同时评估所有生物标志物可增加识别目标变异的机会。
了解有关RET融合的更多内容

NSCLC中RET突变的检测方法
NSCLC的生物标志物在不断发展,随着靶向RET突变肿瘤的新疗法的出现,RET突变检测变得越来越重要。参与本次网络研讨会,医学博士Jaclyn Hechtman将与您分享更多信息。

RET融合的靶向治疗
新型高选择性RET靶向药物已在RET驱动的NSCLC中进行了试验,推进了靶向治疗的发展。通过信息图深入了解这些发现。

患者成功案例:打败IV期肺癌
AJ Patel原本只剩六个月的生命。但8年后,他跟我们分享了生物标志物检测是如何帮助他逆天改命的。
参考文献
- Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients. Clin Cancer Res. 2017;23(8):1988-1997. doi:10.1158/1078-0432.CCR-16-1679
- Drilon A, Hu ZI, Lai GGY, Tan DSW. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15(3):150. doi:10.1038/nrclinonc.2017.188
- Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann Oncol. 2018;29(6):1394-1401. doi:10.1093/annonc/mdy090
- Su X, He C, Ma J, et al. RET/PTC Rearrangements Are Associated with Elevated Postoperative TSH Levels and Multifocal Lesions in Papillary Thyroid Cancer without Concomitant Thyroid Benign Disease. PLoS One. 2016;11(11):e0165596. Published 2016 Nov 1. doi:10.1371/journal.pone.0165596
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378-381. Published 2012 Feb 12. doi:10.1038/nm.2658
- Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18(3):382-384. Published 2012 Feb 12. doi:10.1038/nm.2673
- Ibáñez CF. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb Perspect Biol. 2013;5(2):a009134. doi:10.1101/cshperspect.a009134
- Wang X. Structural studies of GDNF family ligands with their receptors-Insights into ligand recognition and activation of receptor tyrosine kinase RET. Biochem Biophys Acta. 2013;1834:2205-2212
- de Graaff E, Srinivas S, Kilkenny C, et al. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001;15(18):2433‐2444. doi:10.1101/gad.205001
- International Agency on Cancer. World Health Organization. Global Cancer Observatory website. Accessed June 24, 2020. https://gco.iarc.fr/
- Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14(3):173-186. doi:10.1038/nrc3680
- Li AY, McCusker MG, Russo A, et al. RET fusions in solid tumors. Cancer Treat Rev. 2019;81:101911. doi:10.1016/j.ctrv.2019.101911
- Gautschi, O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35:1403-1410. doi:10.1200/JCO.2016.70.9352
- Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients [published correction appears in Nat Med. 2017 Aug 4;23 (8):1004]. Nat Med. 2017;23(6):703-713. doi:10.1038/nm.4333